The growth in the field of polyketides has advanced at such a pace that no short review can hope to be comprehensive. Indeed the September 1997 issue of Chemical Reviews is devoted completely to discussion of a few representative polyketides and polypeptides.
Microorganisms make a wealth
of unusual metabolites that have a secondary role in the organism's ontogeny,
such as self-defence, aggression, or even communication, as the need arises.
Polyketides are such a group of
secondary metabolites, exhibiting remarkable diversity both in terms of
their structure and function. Polyketide natural
products are known to possess a wealth of pharmacologically important activities,
including antimicrobial, antifungal, antiparasitic, antitumor and agrochemical
properties. These metabolites are ubiquitous in distribution and have been
reported from organisms as diverse as bacteria, fungi, plants, insects,
dinoflagellates, mollusks and sponges. The wide spectrum of acticvity of
polyketides makes them economically, clinically and industrially the most
sought after molecules. Many polyketide products are well-known compounds
such as Erythromycin A, a broad spectrum macrolide antibiotic, the antihelmintic
agent avermectin or the immunosuppresants FK506 and rapamycin. Oleandomycin,
rifamycin, lovastatin, oxytetracycline and reserveratrol are a few more
of the thousands of polyketides discovered so far. Polyketides are
usually categorised on the basis of their chemical structures.
Here are some examples of the
myriad chemical structures a polyketide can take:
Figure 1
Synthesis and Asssembly of polyketides
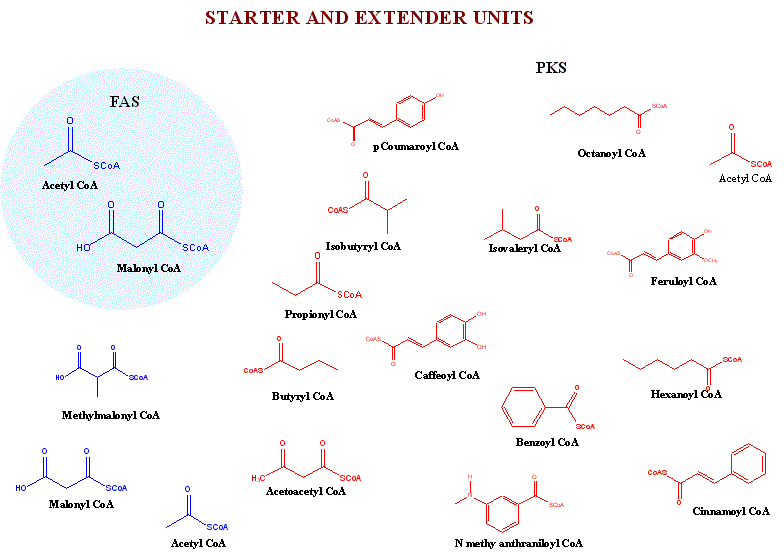
Polyketides are synthesized by sequential reactions catalysed by a collection of enzyme activities called polyketide synthases (PKSs). These are large multienzyme protein complexes that contain a coordinated group of active sites. The biosynthesis occurs in a stepwise manner from simple 2-, 3-, 4-carbon building blocks such as acetyl-CoA, propionyl CoA, butyryl-CoA and their activated derivatives, malonyl-, methylmalonyl- and ethylmalonyl-CoA.
Figure 2
The key chain-building step of polyketide biosyhnthesis is a decarboxylative condensation analogous to the chain elongation step of classical fatty acid biosynthesis which occurs in the following well-understood way:
Figure 3
Unlike fatty acid biosynthesis, however, in which each successive chain elongation step is followed by a fixed sequence of ketoreduction, dehydration and enoyl reduction as shown above, the individual chain elongation intermediates of polyketide biosynthesis undergo all, some, or none of these functional group modifications, resulting in a striking level of chemical complexiyty in the products. Additional degrees of complexity arise from the use of different starter units and chain elongation units as well as the generation of new stereo-isomers.
Types of Polyketide Synthases (PKSs)
At least three architecturally different types of PKSs have been discovered in the microbial world.
Type I systems consist of very large multifuctional poroteins which can be either processive ( for example the unique modular systems responsible for synthesis of macrolides like erythromycin, rapamycin, rifamycin etc.) or iterative (for example the lovastatin nonaketide synthase shown below). Iterative Type I synthases are analogous to vertebrate fatty acid synthases. These are typically involved in the biosynthesis of fungal polyketides such as 6-methylsalicylic acid and aflatoxin. These PKSs are large multidomain proteins carrying all the active sites required for polyketide biosynthesis.
Figure 4
The iterative Type II systems consist of complexes of mono-functional proteins exemplified by the actinorhodin PKS from Streptomyces coelicolor. In these synthases, active sites are distributed among several smaller, typically monofunctional polypeptides. Type II synthases catalyse the formation of compounds that require aromatization and cyclization, but not extensive reduction or reduction/dehydration cycles. These PKSs are analogous to bacterial Fatty Acid Synthases and are involved in the biosynthesis of bacterial aromatic natural products such as actinorhodin, tetracenomycin and doxorubicin. The following cartoon depicts the hypothetical "dynamic" model of an aromatic polyketide synthase:
Figure 5
Type III polyketide synthases are responsible for the synthesis of chalcones and stilbenes in plants and polyhydroxy phenols in bacteria. Chalcone synthase like proteins are comparatively small proteins with a single polypeptide chain and are involved in the biosynthesis of precursors for flavonoids. Unlike all other PKSs, these proteins do not have a phosphopantetheinyl (P-Pant) arm on which the growing polyketide chains are tethered.
Figure 6
Modular polyketide synthases
Modular PKSs constitute a unique
class of Type I polyketide synthases. Each of these proteins consists of
multiple active domains organised into modules. Each module is responsible
for the construction of a carbon-carbon bond, via the decarboxylative condensation
of a ketide extender unit with the growing polyketide chain, followed by
a programmed reductive cycle. In addition there is a loading module in
most PKSs for obtaining the starter unit at the front of module 1 and a
thioesterase responsible for unloading the product at the end of the last
module. The biosynthesis of 6-deoxyerythronolide B (6-dEB) has become the
text-book example for modular polyketide synthases. In Saccaropolyspora
erythrae, it is converted to the biologically active erythromycin by hydroxylation
followed by addition of sugars. This example has been shown in the image
below.
The observation that there is
a separate functional domain for each and every transformation required
to produce a macrolide showed that for the modular PKSs, the programming
of the PKS is achieved through a production-line approach.
Figure 7
The various domains making
up a polyketide synthase
Component domains of polyketides
consist of acyl-transferases (AT) for the loading of starter, extender
and intermediate acyl units; acyl carrier proteins (ACP) which hold the
growing macrolide as a thiol ester; b-keto-acyl synthases (KS) which catalyse
chain extension; b-keto reductases (KR) responsible for the first reduction
to an alochol functionality; dehydratases (DH)which eliminate water to
give an unsaturated thiolester; enoyl reductases (ER) which catalyse the
final reduction to full saturation; and finally a thiolesterase (TE) to
catalyse macrolide release and cyclisation.
Figure 8
The linker hypothesis
Proposed first by Gokhale et
al., (Science, 2000), this hypothesis states that linkers, which can be
defined as the amino acid stretches joining various domains in a modular
polyketide synthase, play a crucial role in the establishment of structural
and functional assembly of these multimodular proteins. It is believed
that these dynamic linkers establish communication by directing the correlated
movements of various domains. Two categories of linkers have been postulated:
linkers that connect covalently connected modules (intra-polypeptide linkers
e.g, between modules 1 and 2 of DEBS) and linkers between modules that
are present on two different polypeptides (inter-polypeptide linkers e.g.,
between modules 2 and 3 of DBES).
The following figure explains
the concept of linkers in a typical modular system:
Figure 9
Scope of polyketide research...
The enzymes that govern the assembly
of polyketides by microorganisms, are receiving increasing attention as
access to them has improved through molecular genetic methods. An explosion
of discoveries and technological innovations has enhanced our capacity
to "mutate" the structure of natural products using heuristics and procedures
that are analogous to those routinely used in the generation of structurally
altered nucleic acids and proteins. Some hurdles exist in the expansion
of a biologist's synthetic capabilities but these barriers can be overcome
by further research. The biological, biochemical, and medical implications
would be immense, specially towards engineering artificial assemblies for
production of novel biotherapeutics. Although organic chemists had conducted
the primary research, geneticists and molecular biologists have completely
transformed this field. Genetic manipulation techniques have resulted in
the identification, cloning, sequencing and functional analyses of several
polyketide biosynthetic pathways. Empirical gene fusion approaches have
led to the biosynthesis of diverse unnatural natural products revealing
the versatility and combinatorial potential of PKSs. With our advancement
in the understanding of the molecular mechanistic basis of polyketide machinery,
it should be possible to rationally alter the PKS genes. Figure 10 is an
example of a successful inter-polypeptide chain transfer between fused
modules of
erythromycin and rifamycin polyketide
synthases.
Figure 10
Excerpts(and/or Figures) taken from
#Khosla C. et al. (1999) Annu. Rev. Biochem.
68:219-253
#Cox R. J. (2000) Annu. Rep. Prog. Chem.,
Sect. B, 96, 231-258
#Gokhale R. S and Tuteja D. (2001) Biotechnology,
10, 341-372
(Also Figures 1, 8 and 7)
Figures 2 and 3 taken with thanks from Ms Yogyata
(NII)
Figure 10 from Gokhale R.S, Tsuji S.Y, Cane D.E and Khosla C. (1999) Science, Vol 284, 482-485